Background
Teclistamab was recently approved as the first-in-class B-cell maturation antigen (BCMA) x CD3 bispecific therapy with personalized weight-based dosing for relapsed or refractory multiple myeloma (MM). Given its novelty, there are immediate needs for data based on real-world (RW) practices to optimize operational processes and improve experiences while ensuring patient safety in the RW. The aims of this study were to describe how US clinicians with early RW experience with teclistamab (1) manage the step-up dosing (SUD) and transition of care (ToC) to and from referring sites; (2) monitor, manage, and prevent adverse events (AEs), i.e., cytokine release syndrome (CRS), infections, and immune effector cell-associated neurotoxicity syndrome (ICANS); and (3) perceive desired future care models for optimal patient care.
Methods
Between April and July 2023, 60-minute, 1-on-1 in-depth structured interviews were conducted virtually with hematologists and oncologists who had treated patients with teclistamab in a RW setting. Interviews elicited clinicians' current SUD and ToC processes in their practices, AE management, and perspectives on SUD model evolution. A subsequent 90-minute roundtable discussion was held in June 2023 to discuss interview findings.
Results
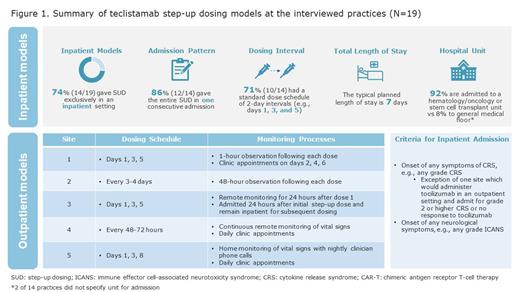
A total of 20 clinicians representing 19 practices across 14 states completed interviews: 84% (16/19 practices) treated >50 patients with MM monthly, 69% had treated >10 patients with teclistamab, and 90% were academic medical centers. Of these interviewees, 10 participated in the roundtable discussion, joined by 3 community clinicians without teclistamab experience. Among the 19 interviewed practices, 14 (74%) were administering SUD exclusively in inpatient settings. This was being done predominantly (86%; 12/14) in 1 continuous admission with a planned length of stay of 7 days, and with 71% (10/14) following a day 1-3-5 dosing schedule. Five practices reported using outpatient models (Figure 1). The sophistication of outpatient models varied and depended on multiple factors at the practice level, including prior outpatient cellular therapy experience, staffing/facility structure, patient volume, and outpatient monitoring capability.
Most (95%) of the interviewed practices initiated teclistamab for patients referred from community practices; 53% transferred at least 1 patient back to a referring practice to continue teclistamab. The time to transfer ranged from 2 weeks to 2 months to (1) ensure that patients were responding to and tolerating treatment prior to transfer and (2) recover any financial loss from inpatient SUD. Community clinicians preferred to resume care of their patients once patients were deemed clinically stable.
More than half (68%) of practices reported leveraging existing CAR-T protocols for AE monitoring and management. Patients were typically monitored for CRS every 4 hours for vital signs and daily for complete blood count, comprehensive metabolic panel, C-reactive protein, and ferritin. CRS prophylaxis with tocilizumab was reported by 11% of practices. 74% (14/19) of practices used tocilizumab for treatment of grade 2 or higher CRS. 94% (17/18) of reporting practices utilized intravenous immunoglobulin for primary infection prophylaxis, typically at a dose of 0.4 g/kg every 4-8 weeks to maintain an IgG level above 400 mg/dL. ICANS risk was considered low, and corticosteroids were the preferred treatment for ICANS. Payer reimbursement, treatment availability and lack of approved indications were noted as barriers for certain prophylactic treatments.
Clinicians indicated a desire to administer SUD in an outpatient setting for patient convenience and to reduce healthcare resource use. Comorbidities, tumor burden, and caregiver support were key patient-level factors to be considered for outpatient SUD. Remote patient monitoring was a potential solution to enhance the outpatient SUD administration.
Conclusions
The results indicated diverse RW administration and patient management strategies in practices with early familiarity with teclistamab, and consolidated practice-based experiences to help inform clinicians employing this innovative therapy. As patient care models evolve, outpatient or community-based SUD may become more common in the future. Ongoing evidence generation on RW treatment outcomes of various SUD models and AE management strategies is warranted.
Disclosures
Derman:Janssen: Consultancy, Honoraria; COTA Healthcare: Consultancy. Roach:Janssen Scientific Affairs: Research Funding. Lin:Janssen Scientific Affairs, LLC.: Current Employment, Current equity holder in publicly-traded company. Wu:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Murphy:Janssen Scientific Affairs: Research Funding. Kim:Janssen Scientific Affairs: Current Employment. Doyle:Janssen: Current Employment, Current equity holder in publicly-traded company. Prood:Janssen Scientific Affairs: Research Funding; PRECISIONheor: Other: Consulting or Advisory role; Research Funding; Travel, Accomodations, or Expenses. Fowler:Janssen: Current Employment. Marshall:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Jamaleddine:Janssen Scientific Affairs: Research Funding. Paner-Straseviciute:Janssen Global Services: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal